Brazil Strengthens Pharmaceutical Cold Chain Logistics Regulations
Release time:2025-03-19
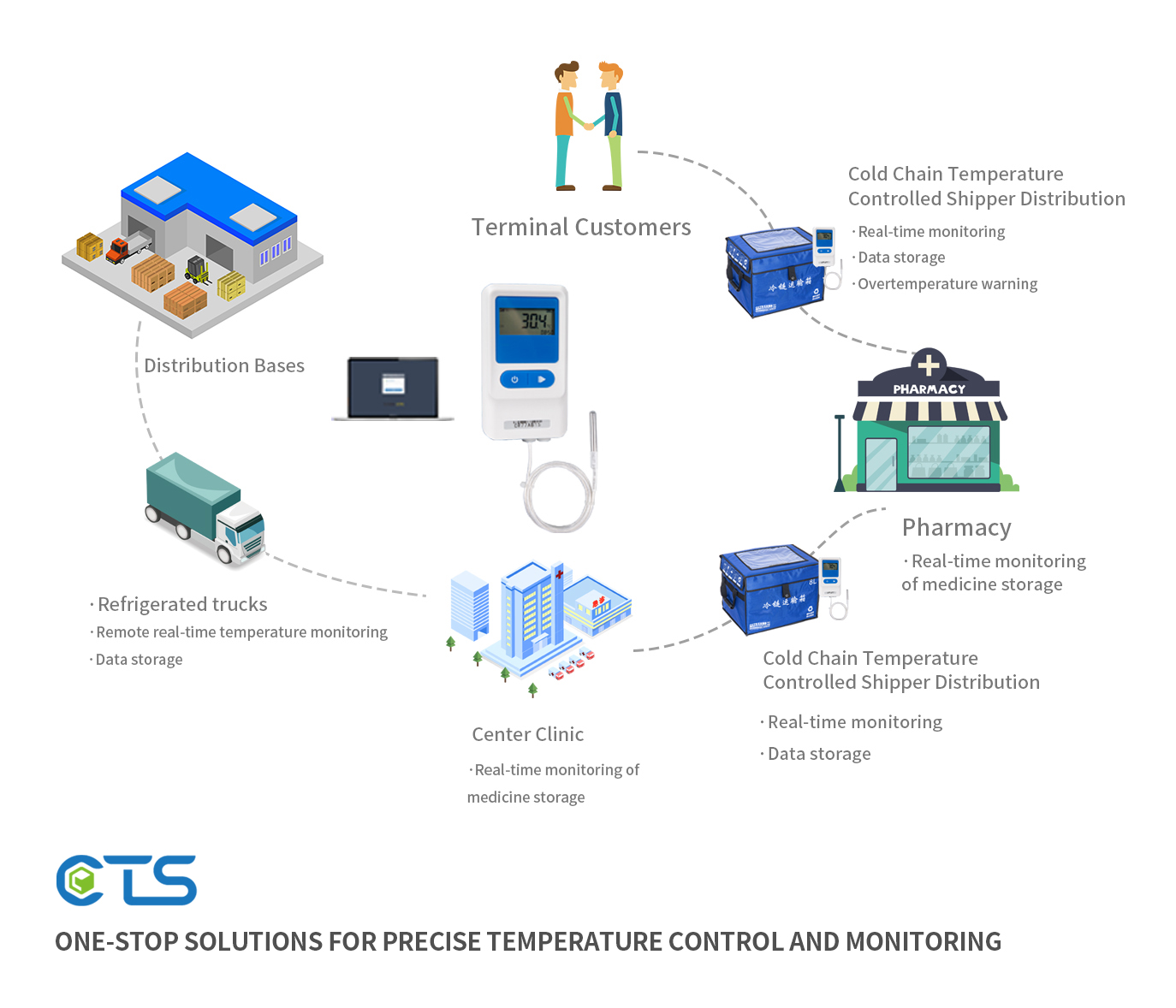
Recently, Brazil's health regulatory agency ANVISA has strengthened its supervision of pharmaceutical cold chain logistics. Pharmaceutical cold chain logistics enterprises are required to be equipped with more advanced temperature monitoring equipment to ensure that the temperature of drugs remains within the specified range during transportation and storage. At the same time, the technical standards for cold chain transportation vehicles have also been updated. Vehicles are required to have better refrigeration and heat preservation performance, as well as a more reliable backup power system, to prevent temperature out - of - control during transportation due to power failures and other reasons. This series of measures aims to improve the overall quality and safety of Brazil's pharmaceutical cold chain logistics and ensure the safety of people's medication. Currently, many Brazilian pharmaceutical companies and logistics suppliers are actively responding to these regulations and increasing their investment in cold chain equipment and technology.